
【引用格式】李宇诚,Julien Baker.轻度认知功能障碍的非药物干预——体育锻炼[J]. 中国神经精神疾病杂志,2023,49(2):65-75.
【Cite this article】LI Y C,Julien Baker. Physical exercise: a promising non-pharmacological intervention for patients with mild cognitive impairment[J]. Chin J Nervous Mental Dis,2023,49(2):65-75.
作者简介

朱利安贝克教授,哲学博士及科学博士,现任香港浸会 大学运动、体育及健康学系主任。人口健康和医学信息学研究组负责人。 在同行评议期刊上发表了 650 多篇文章。生理学学会、皇家生物学会、生物学会和临床研究机构会士,美国生理学会和生物学研究学会(SSOB)成员,英国药理学会和美国实验生物学学会联合会(FASEB)会员。
Professor Julien Baker PhD, DSc, is Head of the Sport, Physical Education and Health Department at Hong Kong Baptist University. He is also the director of the Population Health and Medical Informatics Research group. He has pub⁃ lished over 650 papers in peer reviewed journals. Professor Baker has Fellow⁃ ships with the Physiological Society, the Royal Society of Biology, the Biological Society and the Institute of Clinical Research. Professor Baker is also a member of the American Physiological Society, and the Society for the Study of Biology (SSOB). In addition, he has membership of the British Pharmacological Society, and the Federation of American Societies for Experimental Biology (FASEB).
轻度认知功能障碍的非药物干预
—— ——体育锻炼
李宇诚Julien Baker
中山大学附属第一医院神经科;
香港浸会大学(Hong Kong Baptist University)
摘 要轻度认知功能障碍(mild cognitive impairment,MCI)发生于阿尔茨海默病(Alzheimer disease,AD)的早期阶段,非药物干预手段是改善MCI患者认知功能的途径之一。在非药物干预方式中,体育锻炼正被越来越多人所关注。本文就体育锻炼对MCI患者认知功能改善做一综述,为MCI的治疗与管理提供思路。体育训练可分为有氧训练与阻力训练,两种训练方式均可以改善MCI患者的整体认知功能,但不同运动对不同认知领域的作用仍不明确。此外,体育训练联合其他非药物干预方式对MCI患者的认知功能改善也展现出一定潜力。另一方面,运动改善认知功能的机制与神经发生、神经元存活和突触可塑性有关,这既可以在分子细胞水平解读,也可以从器官等宏观角度分析。最后,本文指出进行同质性研究、运动处方的探索与远程运动管理的实施作为MCI与运动相关研究的可能方向。
关键词
轻度认知功能障碍;阿尔茨海默病;体育锻炼;非药物干预;认知
阿尔茨海默病(Alzheimer disease,AD)是导致痴呆的最常见原因,也是一个主要的公共健康问题[1]。AD引起的轻度认知功能障碍(mild cognitive impairment,MCI)发生在疾病的早期,此阶段存在明显的认知功能障碍迹像,但并不影响日常活动。尽管AD尚没有治愈的方法,但越来越多的证据表明,体育活动或许能够降低从MCI到痴呆的转换率[2]。
体育锻炼旨在改善健康、提升生活质量。运动训练可以分成两种不同的类型:有氧训练和阻力训练[3]。有氧锻炼可以提高有氧工作能力,进而增强心肺功能,减少脑血管疾病、运动障碍和残疾的发生[4]。它包括很多常见的运动形式,如健步走、慢跑、水中有氧运动、游泳、跳舞和骑自行车[5]。此外,当运动强度达到有氧供能系统的要求时,太极拳、瑜伽等几种特殊形式的运动也可归为有氧运动。另一方面,阻力训练通常被应用于从事运动相关工作的群体,或者其他想要诱导神经肌肉适应、以增加肌肉量和增强功能的个人[6]。这些运动在本质上是无氧的,提供能量的燃料主要来自无氧糖酵解和三磷酸腺苷的分解。这种能量供应非常迅速,促进肌肉短时间内产生强力收缩[7]。有氧代谢则随着运动持续时间的延长和强度的降低而增强。
1运动锻炼与联合干预改善MCI患者的认知功能
有氧锻炼和阻力训练都能提高社区健康老年人的认知能力,并改善MCI患者的整体认知能力[2, 8-12 ]。然而,正向的结果与运动强度、持续时间和频率高度相关。例如,短时间、高频率的训练可能对认知改善有更大的效果,因为短时间的训练可能更不容易引起疲劳,这种情况与锻炼的能力和动机有关[13]。另一方面,由于研究的异质性,运动对不同认知领域的作用也存在差异[10, 14]。
TALAR等[14]在一项meta分析中得出结论,有氧运动对工作记忆和执行功能方面的影响没有统计学意义。与之对比,在一项临床试验中,有氧锻炼组和拉伸运动组的参与者进行每周4天、每次45~60 min的高强度活动,持续6个月。两组在多任务处理、认知灵活性、信息处理效率和选择性注意等执行控制过程中均取得较好的效果[11]。此外,有证据表明,有氧运动提高了即时回忆和延迟回忆能力,但对意向、执行能力、语言流畅性或视觉空间功能等认知领域没有影响[10]。
类似的,阻力训练可以提高MCI患者的执行功能和联想记忆能力[8-9 ]。有趣的是,ZHU等[15]发现进行等距握力练习对认知表现有长期的有益影响,但需要严谨的方法去评估练习的可行性和有效性。瑜伽是一种典型的有氧运动,它主要关注大脑、身体和精神之间的相互作用。EYRE等[16]研究发现,昆达里尼瑜伽对记忆力改善的效果与认知训练类似,并且对执行功能和情绪症状具有额外的益处。此外,太极拳是中国传统体育运动之一,有证据表明,它可以改善老年MCI患者的总体认知表现、记忆、注意力、语言和执行功能[17]。这些引起争议的发现表明,体育锻炼和认知之间存在复杂的关系。鉴于有氧运动的类型和(或)设置各不相同,对认知领域的确切影响需要未来更多科学的原始研究进一步明确。
从理论上讲,不同干预措施相结合可能会对MCI患者认知功能提供更好的保护。事实上,有氧运动和认知训练的结合已经被证明可以减少视觉记忆和语言流畅性的衰退,并改善执行功能[18]。一项meta分析还显示,认知-体育锻炼联合干预对MCI或痴呆老年人的整体认知功能具有积极的小到中等效应(SMD=0.32 [0.17; 0.47],P<0.00),并且各研究之间无显著异质性[19]。然而,不合适的干预组合可能效果不佳。例如,接受全训练量阻力训练和认知训练的患者在执行和总体认知方面的表现,明显较接受单独的渐进式阻力训练的患者差[20]。这可能是由于联合干预会产生过大的压力,对精神和身体同时造成挑战,患者因此可能更少参与家庭或社区活动。另外,组合干预可能会导致副作用,抑制而非促进神经可塑性和认知效益[20]。进一步来说,运动处方的提供和开具应该基于每例患者的具体情况,这将保证为每个人推荐合适强度的运动方式。
另一个有趣的研究方向是将体育锻炼与经颅直流电刺激(transcranial direct current stimulation,DCS)、经颅磁刺激(transcranial magnetic stimulation,TMS)或其他非侵入性手段结合来改善认知功能。尽管这一领域的相关文献有限,但新的证据表明,在改善认知相关的双任务步行方面,阳极tDCS联合太极训练效果优于假刺激联合太极训练[21]。
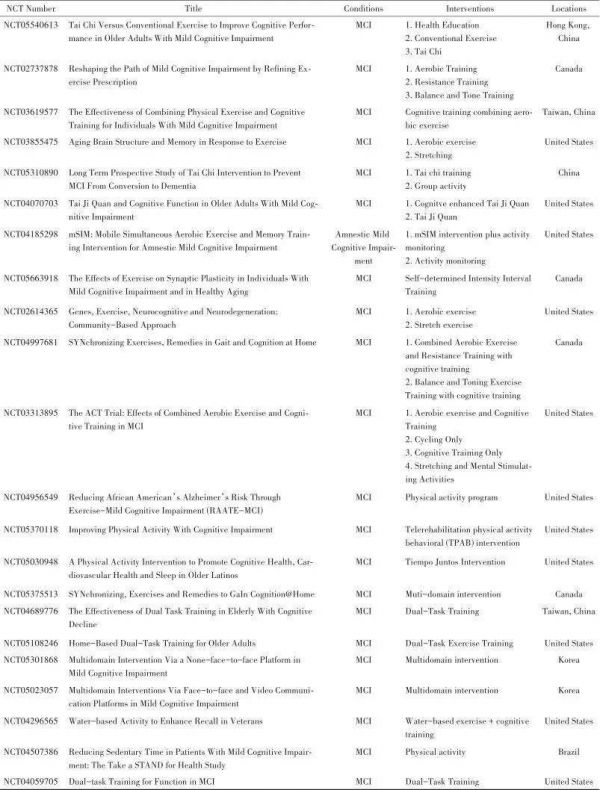
目前正在进行的关于运动改善MCI患者认知功能的临床研究见表1。
表1正在进行的关于运动对MCI患者认知改善的临床研究

注:NCT,国家临床试验;MCI,轻度认知功能障碍;aMCI,遗忘型轻度认知功能障碍;mSIM,基于移动的同步运动和记忆技能训练计划;ACT,有氧练习联合认知训练。
2运动改善MCI患者认知功能的机制
运动改善认知的内在机制尚未完全阐明。然而,运动诱导的大脑获益很大程度上与神经发生、神经元存活和突触可塑性有关。事实上,体育锻炼可能促进脑源性神经营养因子(brain derived neurotrophic factor,BDNF)等神经营养因子的产生、调节血清素和犬尿氨酸代谢、诱导肌脑交互作用中的肌细胞因子、增强抗炎反应和线粒体介导的调节作用[22]。然而,一项meta分析的亚组分析证实,运动干预增加了MCI患者血清中BDNF水平,但趋势不显著(SMD=1.07;95%CI:-0.14~2.28;I²= 86%;P=0.080)。产生这种不确定结果的原因可能是统计效力低,也可能是认知功能受损导致MCI患者按要求进行运动干预的能力降低[23],这也是与个人运动处方计划相关的因素。另一个有趣的领域是线粒体和肌-脑轴的作用,BURTSCHER等[24]证实,在体育锻炼后,骨骼肌中的线粒体会被激活,并分泌包括肌细胞因子、代谢产物和微小核糖核酸在内的循环因子,直接或间接地改善大脑线粒体健康,并可能使认知达到更好的状态。此外,有氧系统在决定长时间高强度运动的表现方面起着重要作用[7]。过度活跃的有氧系统结合无氧糖酵解可能触发蛋白质的表达,这些蛋白将参与线粒体的产生和能量的生产,激活的有氧系统和糖酵解也会诱导氧化磷酸化相关蛋白的翻译,并促进骨骼肌中ATP的生成[24]。此外,剧烈运动会引起循环干细胞和祖细胞数量的增加,并同时启动内源性神经干细胞的动员[25-26 ]。这些分子或细胞通路可以直接促进海马神经元的活化和提高其可塑性,也可以通过增加脑血容量和毛细血管密度、改善全身情况等间接影响神经元[22, 27]。有证据表明,认知功能下降患者在接受体育训练后海马体积增大[3]。
另一方面,β-淀粉样蛋白(β-amyloid,Aβ)斑块和细胞内神经纤维缠结的累积、tau蛋白的过度磷酸化是AD的主要特征,并被认为是导致脑体积减小和脑功能下降的原因[28]。来自动物研究的证据表明,运动可能有助于抑制Aβ产生和促进大脑中Aβ清除[28]。同时,有证据表明,体育锻炼水平可能会影响大脑、脑脊液和人体血液中的Aβ水平[29]。LIANG等[30]发现,达到美国心脏协会指导方针即7.5代谢当量小时/周运动的个体(n=69),表现出明显较低的匹兹堡化合物B(Pittsburgh compound B,PiB,它是硫黄素T的类似物,是淀粉样蛋白成像最常用的示踪剂)结合水平和较高的脑脊液Aβ42水平,提示一种更健康的淀粉样蛋白谱。载脂蛋白E(apolipoprotein E,APOE)ε4等位基因是散发性AD最强的已知遗传危险因素[31]。它与Aβ聚集率升高、大脑Aβ清除减少、认知能力下降和神经易损性增加有关[28]。先前的研究表明,高水平体育活动可能会降低APOE ε4载体带来的Aβ沉积风险的增加[32]。此外,BROWN等[33]证实,在报告最高体力活动水平且认知功能正常的老年人中,正电子发射扫描量化的tau蛋白水平较低。然而,随着关于Aβ和tau的检测技术发展,我们希望有更多专门设计的、以运动为重点的随机对照研究,并把运用金标准测量的Aβ和tau作为结果。此外,探明运动的神经保护机制也有助于药物的开发(图1)。
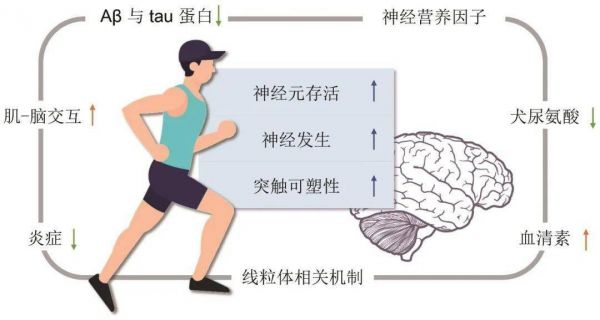
图1运动改善MCI患者认知功能的可能分子和细胞机制 注:Aβ,β淀粉样蛋白。
不同形式的体育锻炼可以通过作用于大脑不同区域来提高认知能力。据报道,有氧运动与海马的激活有关,而瑜伽则激活负责整合思想和情绪的额叶和岛叶。此外,举重通过对前额叶皮层作用来提高执行功能。在一项纵向研究中,对接受6周运动训练的年轻成人进行功能和结构MRI研究,结果显示额顶叶的网络连通性增加,并与认知功能的改善一致。灰质结构改变也与前额叶和辅助运动区的功能连通性改变密切相关[34]。此外,阻力训练已经被发现可以调节皮质脊髓的适应力和减少白质病变体积[6]。总的来说,这些证据表明,一般性运动可能不能充分调动运动在MCI中的治疗潜力,包括内定模态网络、额顶网络和额叶执行网络等脑网络是认知衰退干预的潜在靶点[35]。基于个体认知域损伤的精确运动处方是可取的,这可能需要不同强度、持续时间和休息时间的锻炼计划,以最大限度地发挥运动对大脑健康的促进作用。
3展望
远程锻炼指导或远程监督体育锻炼处于对抗认知衰退的新前线。MCI患者的短期运动干预通常在医院的健康中心进行,鉴于持续的运动可以提供长期保护作用,对MCI患者来说,长期的运动计划是必要的。然而,长期坚持有氧运动对MCI患者来说是一项挑战,因为这需要收集生理数据,并测量运动强度。新的文献表明,远程医疗是家庭认知训练的理想手段。在家锻炼可能是一个可行的选择,因为它方便时间安排,有良好的可接受性。在以家庭为基础的环境中,远程锻炼项目可以在家庭或附近社区的支持下,由专业教练提供远程监督,从而提高患者的依从性和提高锻炼的有效性。例如,Vivifrail项目是一个以家庭为基础的锻炼项目,其重点是根据老年人的功能能力制定个性化的多组分锻炼处方,经过12周的训练,试验组和对照组的认知功能和握力具有显著差异[36]。另一方面,较低的握力已被发现与较高的全因痴呆发病率和死亡率相关,独立于重要的混杂因素[37]。总之,握力可能是一种很有希望的评估体育锻炼效果的方法,也可能是认知能力下降的预测指标。综上所述,未来的研究需要考虑不同运动强度和运动时长的影响、运动/休息恢复比以及更合适的测量方法。此外,与单纯的步行、跑步或游泳相比,在运动中含有技巧元素的锻炼方式可能会带来更大的益处。
(参考文献同英文版)
1Exercise and combined intervention can improve cognitive function in patients with MCI
Alzheimer disease (AD) is the most common cause of dementia and a major public health problem[1]. Mild cognitive impairment (MCI) due to AD is a prodromal stage of AD where there are notable signs of cognitive impairment. However, MCI does not interfere with activities of daily life. Although there is no cure for AD, accumulated evidence suggests that physical activity may reduce the conversion rates from MCI to dementia[2].
Exercise is a form of physical activity aimed to improve health and well-being. There are two distinct types of exercise training: aerobic training and resistance training[3]. Aerobic exercise can improve aerobic capacity, thereby enhancing cardiopulmonary capacity and reducing cerebrovascular disease, mobility limitations and disability[4]. Aerobic exercise includes many commonly practiced exercises such as brisk walking, jogging, water aerobics, swimming, dancing, or bicycle riding[5]. In addition, tai chi, yoga and several special forms of exercises can also be classified as aerobic exercise when the intensity of exercise meets the required demands of the aerobic exergy supply system. On the other hand, resistance exercise is usually performed by athletic populations and individuals who would like to induce neuromuscular adaptations to increase muscle size and performance[6]. These types of exercises are anaerobic in nature, and the fuel for energy provision comes predominantly from anaerobic glycolysis and the breakdown of adenosine triphosphate (ATP). The energy supply from these metabolic pathways is very rapid and facilitates strong powerful contractions over short time periods[7]. Aerobic metabolism increases as the duration of the activity becomes longer and decreases in intensity occur.
Both aerobic training and resistance training enhance cognitive performance in healthy, community-dwelling seniors and improve the overall cognitive performance of patients with MCI[2, 8-12 ]. However, the positive outcomes are highly associated with intensity, duration and frequency of exercise. For example, shorter duration training sessions and higher frequencies may have greater cognitive effects in that short sessions may induce less fatigue, the condition associated with the ability and motivation to exercise[13]. On the other hand, the action of exercise on the different domains of cognition remains diverse due to the heterogeneity of research[10, 14].
In a meta-analysis, TALAR et al. concluded that there were no statistically significant effects of aerobic exercise on working memory and executive function[14]. In contrast, in a clinical trial which participants in both the aerobic and stretching groups performed high-intensity activity routines 4 d/wk for 45 to 60 minutes per session for 6 months demonstrated favorable results. Both groups achieved a better executive control processes of multitasking, cognitive flexibility, information processing efficiency, and selective attention[11]. Moreover, there is evidence that aerobic exercise improves immediate recall ability as well as delayed recall ability but not cognitive domain of intention, executive ability, verbal fluency or visuospatial function[10].
Similarly, resistance training may promote executive function and associative memory in patients with MCI[8-9 ]. Interestingly, ZHU et al. discovered a trend that implementation of isometric handgrip exercise has a beneficial chronic effect on cognitive performance, while the rigorous methodological approach is needed to evaluate the feasibility and effectiveness of the exercise[15]. Yoga is a typical aerobic exercise with a focus on interactions among the brain, body and mind. Eyre’s study found that Kundalini yoga had similar improvements on memory during cognitive training, with additional beneficial effects on executive functioning and mood symptoms[16]. Additionally, Tai chi, as one of the traditional Chinese sports, showed evidence on improving cognitive performance, memory, attention, language and executive functions in older adults with MCI[17]. The controversial findings suggest that there are complex relationships between physical exercise and cognition. Given that the types and/or settings of aerobic exercise vary from one another, the precise effect on the cognition domains require more scientific original studies in the future.
Theoretically, the combination of different interventions may provide better protection on cognition in patients with MCI. Indeed, combinations of aerobic exercise and cognitive training, have been shown to reduce the decline of visual memory and verbal fluency and improve executive function[18]. A meta-analysis also demonstrated a positive small-to-medium effect of combined cognitive-physical exercise interventions on global cognitive function in older adults with MCI or dementia (SMD=0.32 [0.17;0.47], P<0.00),without significant heterogeneity across the studies[19]. However, inadequate combinations may be less effective. For instance, patients receiving full doses of resistance training and cognitive training performed significantly poorer compared with isolated progressive resistance training on executive and global domains[20]. This may be due to the excessive stress of the combined intervention, which was both mentally and physically challenging. As a result, patients may be less engaged with home or community-based activities. Alternatively, combinations may induce side effects that inhibit rather than promote neural plasticity and cognitive benefits[20]. Further to this, the exercise training mode should be provided and prescribed to the patients on an individual basis. This will ensure that the modalities of exercise are prescribed at the correct intensities for individual subjects.
One interesting research direction would be to combine physical exercise with transcranial direct current stimulation (tDCS) or transcranial magnetic stimulation (TMS), non-invasive tools to enhance cognition. Although the relevant literature in this area is limited, emerging evidence suggests that concurrent anodal tDCS and Taichi training was superior to a sham stimulation with Taichi training in improving cognitive dual-task walking[21].
Ongoing clinical studies on exercise improving cognitive function in patients with MCI are shown in Table 1.
2The mechanisms of exercise improving cognitive function in MCI patients
The underlying mechanisms have not yet been fully elucidated. However, exercise-induced brain benefits are largely related to neurogenesis, neuronal survival, and synaptic plasticity. Indeed, physical exercise may promote the production of neurotrophins such as brain-derived neurotrophic factor (BDNF), modulate serotonin and kynurenine metabolism, induce myokines in muscle-brain crosstalk, increase anti-Inflammatory response and mitochondrial-mediated regulation[22].
However, subgroup analyses of a meta study confirmed that exercise interventions increased plasma BDNF levels for MCI with a non-significant trend (SMD= 1.07; 95% CI: -0.14~2.28; I²=86%; P=0.080). The indeterminate result might be potentially due to low statistical power, but also to the reduced ability of MCI patients to comply with the prescribed exercise interventions because of their cognitive impairment[23]. This is another factor related to individual exercise preion regimes.
Another interesting field is the role of the mitochondria and the muscle-brain axis. BURTSCHER et al. demonstrated that mitochondria in the skeletal muscle will be activated after physical exercise and secreting circulating factors including myokines, metabolites and microRNAs, which directly and indirectly improve brain mitochondria health, possibly resulting in a better cognitive state[24]. Moreover, the aerobic system plays a significant role in determining performance during high-intensity exercise over long exercise periods[7]. An overactive aerobic system in combination with anaerobic glycolysis may be a trigger to upregulate proteins involved in mitochondrial biogenesis and energy production, to induce the translation of oxidative phosphorylation-linked proteins, and to improve ATP generation in skeletal muscle[24].
Besides, acute exercise elicits an increase in circulating stem and progenitor cell numbers, while the mobilization of endogenous neural stem cells also begins[25-26 ]. Together, these molecular or cellular pathways may directly increase hippocampal neuronal activation and plasticity in turn, or indirectly affect neurons through increases in cerebral blood volume capillary densities, as well as improvement in whole body situation[22, 27]. Increased hippocampal volume has also been observed following physical training in people with cognition decline[3].
On the other hand, beta-amyloid (Aβ) plaques and intracellular accumulation of neurofibrillary tangles, hyperphosphorylated tau proteins, are the primary hallmarks of AD, and are proposed to contribute to a decline in brain volume and function[28]. Comprising evidence from animal studies suggests that exercise may contribute to both the inhibition of Aβ production and enhancement of Aβ clearance in the brain[28]. Meanwhile, there is evidence that physical exercise levels might affect Aβ levels in the brain, cerebrospinal fluid (CSF), and blood in human bodies[29]. LIANG et al. found that individuals (n=69) meeting the American Heart Association guidelines of 7.5 metabolic equivalent (MET) hours/week of exercise showed significantly lower PiB(The Pittsburgh compound B, which is the analogue of thioflavin T, is the most commonly used tracer for amyloid imaging)binding and higher levels of Aβ42in the CSF, indicating a healthier amyloid profile[30]. The Apolipoprotein E (APOE) ε4 allele is the strongest known genetic risk factor for sporadic onset AD[31]. It is associated with higher rates of Aβ aggregation, reduced clearance of Aβ from the brain, increased rate of cognitive decline and neuronal vulnerability[28]. Previous research suggested higher levels of physical activities may lead to mitigating the increased risk of Aβ deposition conferred by APOE ε4 carriage[32]. Besides, BROWN et al. demonstrated lower levels of PET-quantified tau in cognitively normal older adults reporting the highest levels of physical activity[33]. However, as the measurements of Aβ and tau are developing,studies applying gold-standard tau and Aβ measurements as outcome measures in specifically designed exercise-focused randomized controlled trials are expected. Furthermore, identification neuroprotective mechanism by exercise may also contribute to drug development (Figure 1).
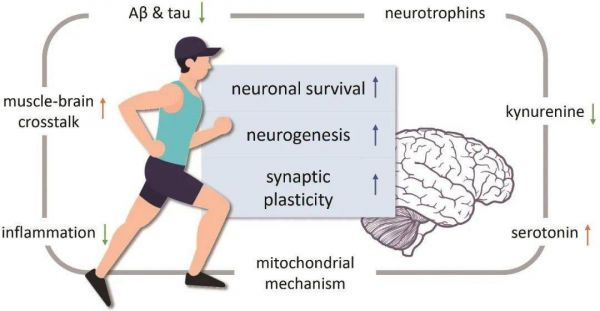
Figure 1Possible molecular and cellular mechanisms of exercise improving cognitive function in MCI patients
Different forms of physical exercise may improve cognitive ability by targeting different areas of the brain. Aerobic exercise has been reported to be associated with the activation of the hippocampus, while Yoga activates the frontal lobe and insula, which are responsible for integrating thoughts and emotions. In addition, lifting weights boosts executive function through the action on the prefrontal cortex. Longitudinal functional and structural MRI study of young adults who accepted a 6-week motor training showed increased fronto-parietal network connectivity in accordance with cognitive performance improvements. The structural gray matter alterations were also tightly correlated with functional connectivity changes in prefrontal and supplementary-motor areas[34]. Moreover, resistance training has been found to modulate corticospinal adaptations and lower white matter lesion volume[6]. Collectively, this evidence suggests that generic exercise may not take full advantage of therapeutic potential of exercise in MCI. Several brain networks including Default Mode Network mainly, Fronto-Parietal Network and Fronto-Executive Network are potential targets for the interventions of cognitive decline[35]. Precision exercise preion based on individual cognitive domain impairment is desirable. This may require exercise regimes of different intensities, durations and rest recovery periods to maximize the full benefits of exercise on brain health.
3Expectations
Tele-exercise or remoted-supervised physical exercise is a new frontline in the battle against cognitive decline. Short-term exercise interventions in MCI people were usually conducted in hospitals health centers. Given that sustainable exercise can provide long-term protective effects, a long-term exercise program is necessary for MCI patients. However, long-term adherence to aerobic exercise is challenging for patients with MCI in that the physiological data needs to be collected and needs to be measured for the establishment of exercise intensity. Home-based Emerging literature has demonstrated that telemedicine is an ideal tool for home-based cognitive training. Home-based exercise may be a feasible option because of its convenient schedule and easy acceptability. In the home-based setting, tele-exercise programs can provide remote supervision by professional trainers while being supported by the family or near communities, thus improving patient adherence and increasing effectiveness of exercise. For instance, the Vivifrail programme was a home-based exercise programme focused on individualized multicomponent exercise preion according to the functional capacity of the older adults. After 12 weeks of training, significant differences were observed for cognitive function and handgrip strength[36]. On the other hand, lower handgrip strength has been found to be associated with a higher risk of all-cause dementia incidence and mortality, independent of important confounding factors[37]. Together, handgrip strength may be a promising measurement for the effect of physical exercise as well as a predictor for cognitive decline. In conclusion, future research needs to consider the effects of different intensities of exercise, the effects of different durations, work to rest recovery ratios and more suitable measurements. Further to this, exercise modalities that have an element of skill in the exercise being performed may provide greater benefits than simply walking, running or swimming.
Table 1Ongoing clinical studies of exercise on cognitive improvement in patients with MCI
1.LI J Q, TAN L, WANG H F, et al. Risk factors for predicting progression from mild cognitive impairment to Alzheimer's disease: a systematic review and meta-analysis of cohort studies[J]. J Neurol Neurosurg Psychiatry, 2016, 87(5): 476-484.
2.LAW C K, LAM F M, CHUNG R C, et al. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: a systematic review[J]. J Physiother, 2020, 66(1): 9-18.
3.TEN BRINKE L F, BOLANDZADEH N, NAGAMATSU L S, et al. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial[J]. Br J Sports Med, 2015, 49(4): 248-254.
4.NEWMAN A B, KUPELIAN V, VISSER M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort[J]. J Gerontol A Biol Sci Med Sci, 2006, 61(1): 72-77.
8.LIU-AMBROSE T, NAGAMATSU L S, GRAF P, et al. Resistance training and executive functions: a 12-month randomized controlled trial[J]. Arch Intern Med, 2010, 170(2): 170-178.
9.NAGAMATSU L S, HANDY T C, HSU C L, et al. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment[J]. Arch Intern Med, 2012, 172(8): 666-668.
10.ZHENG G, XIA R, ZHOU W, et al. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials[J]. Br J Sports Med, 2016, 50(23): 1443-1450.
11.BAKER L D, FRANK L L, FOSTER-SCHUBERT K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial[J]. Arch Neurol, 2010, 67(1): 71-79.
16.EYRE HA, SIDDARTH P, ACEVEDO B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment[J]. Int Psychogeriatr, 2017, 29(4): 557-567.
17.ZHENG G, LIU F, LI S, et al. Tai Chi and the Protection of Cognitive Ability: A Systematic Review of Prospective Studies in Healthy Adults[J]. Am J Prev Med, 2015, 49(1): 89-97.
18.LISSEK V J, BEN ABDALLAH H, PRAETORIUS A, et al. go4cognition: Combined Physiological and Cognitive Intervention in Mild Cognitive Impairment[J]. J Alzheimers Dis, 2022, 89(2): 449-462.
19.KARSSEMEIJER E G A, AARONSON J A, BOSSERS W J, et al. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis[J]. Ageing Res Rev, 2017, 40: 75-83.
20.FIATARONE SINGH M A, GATES N, SAIGAL N, et al. The Study of Mental and Resistance Training (SMART) study-resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial[J]. J Am Med Dir Assoc, 2014, 15(12): 873-880.
22.LIU Y, YAN T, CHU J M, et al. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases[J]. Lab Invest, 2019, 99(7): 943- 957.
23.RUIZ-GONZALEZ D, HERNANDEZ-MARTINEZ A, VALENZUELA P L, et al. Effects of physical exercise on plasma brain-derived neurotrophic factor in neurodegenerative disorders: A systematic review and meta-analysis of randomized controlled trials[J]. Neurosci Biobehav Rev, 2021, 128: 394-405.
24.BURTSCHER J, MILLET G P, PLACE N, et al. The Muscle-Brain Axis and Neurodegenerative Diseases: The Key Role of Mitochondria in Exercise-Induced Neuroprotection[J]. Int J Mol Sci, 2021, 22(12): 6479.
25.SCHMID M, KROPFL J M, SPENGLER C M. Changes in Circulating Stem and Progenitor Cell Numbers Following Acute Exercise in Healthy Human Subjects: a Systematic Review and Meta-analysis[J]. Stem Cell Rev Rep, 2021, 17(4): 1091-1120.
27.TARUMI T, ZHANG R. Cerebral hemodynamics of the aging brain: risk of Alzheimer disease and benefit of aerobic exercise[J]. Front Physiol, 2014, 5: 6.
28.BROWN B M, PEIFFER J, RAINEY-SMITH S R. Exploring the relationship between physical activity, beta-amyloid and tau: A narrative review[J]. Ageing Res Rev, 2019, 50: 9-18.
29.BRINI S, SOHRABI H R, PEIFFER J J, et al. Physical Activity in Preventing Alzheimer's Disease and Cognitive Decline: A Narrative Review[J]. Sports Med, 2018, 48(1): 29-44.
30.LIANG K Y, MINTUN M A, FAGAN A M, et al. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults[J]. Ann Neurol, 2010, 68(3): 311-318.
31.CORDER E H, SAUNDERS A M, STRITTMATTER W J, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families[J]. Science, 1993, 261(5123): 921-923.
32.BROWN B M, PEIFFER J J, TADDEI K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing[J]. Mol Psychiatry, 2013, 18(8): 875-881.
33.BROWN B M, RAINEY-SMITH S R, DORE V, et al. Self-Reported Physical Activity is Associated with Tau Burden Measured by Positron Emission Tomography[J]. J Alzheimers Dis, 2018, 63(4): 1299-1305.
34.TAUBERT M, LOHMANN G, MARGULIES D S, et al. Longterm effects of motor training on resting-state networks and underlying brain structure[J]. Neuroimage, 2011, 57(4): 1492-1498.
35.HUANG P, FANG R, LI B Y, et al. Exercise-Related Changes of Networks in Aging and Mild Cognitive Impairment Brain[J]. Front Aging Neurosci, 2016, 8: 47.
36.CASAS-HERRERO A, SAEZ D E, ASTEASU M L, et al. Effects of Vivifrail multicomponent intervention on functional capacity: a multicentre, randomized controlled trial[J]. J Cachexia Sarcope⁃nia Muscle, 2022, 13(2): 884-893.
37.Esteban-Cornejo I, Ho FK, Petermann-Rocha F, et al. Handgrip strength and all-cause dementia incidence and mortality: find⁃ings from the UK Biobank prospective cohort study[J]. J Cachexia Sarcopenia Muscle, 2022, 13(3): 1514-1525.
Physical exercise: a promising non-pharmacological intervention for patients with mild cognitive impairment
LI Yucheng Julien Baker
Department of Neurology, The First Affiliated Hospital, Sun Yatsen University
Hong Kong Baptist University
Abstract:Mild cognitive impairment (MCI) occurs in the early stage of Alzheimer disease (AD). Non-pharmacological interventions are one of the ways to improve the cognitive function of MCI patients. Among the non-pharmacological interventions, physical exercise is attracting more and more attention. This article reviews the effects of physical exercise on cognitive improvement in MCI patients, so as to provide ideas for the treatment and management of MCI. Physical training can be divided into aerobic training and resistance training. Both training methods can improve the overall cognitive function of MCI patients, but the effects of different exercises on different cognitive domains are still unclear. In addition, physical training combined with other non-pharmacological interventions also shows a certain potential for cognitive improvement in MCI patients. On the other hand, the mechanisms by which exercise improves cognition are related to neurogenesis, neuronal survival, and synaptic plasticity, which can be interpreted both at the molecular cellular level and from a macroscopic perspective such as organ. Finally, this paper provides conducting homogeneous research, exploration of exercise preion and implementation of remote exercise management as possible directions for MCI and exercise related research.
Keywords:Mild cognitive impairment;Alzheimer disease;Physical exercise;Non-pharmacological intervention;Cognition
声明:本文作者享有本文著作权,《中国神经精神疾病杂志》专有本文出版权和信息网络传播权,转载请注明作者与出处。部分图转自网络。
初审:李立
审核:邢世会
审定发布:张为西返回搜狐,查看更多
责任编辑:






